
Development code: IK-01
- Product description/product information
- Treatment strategies for knee cartilage with IK-01
- Status of development
Product description/product information
Collagen matrix containing cultured autologous chondrocytes (development code: IK-01) is a product produced from cells collected from the patient’s own cartilage tissue of the knee joint and then seeded and cultured in a collagen gel. This product features the ability to realize a simple surgical procedure by using a collagen matrix having a reasonable strength as a 3D-scaffold. An investigator-initiated trial with IK-01 was conducted in 2012 in subjects with traumatic knee cartilage damage at the Department of Orthopaedic Surgery, Kove University School of Medicine, and at the Foundation for Biomedical Research and Innovation at Kobe (FBRI). We originally entered into a joint development agreement for IK-01 with FBRI in 2015, and a confirmatory trial was started in 2018, mainly at Department of Orthopaedic Surgery, Kove University School of Medicine, and Department of Orthopaedic Surgery, Hirosaki University School of Medicine, and has been completed. In addition, more recently, a clinical study in subjects with knee osteoarthritis has been undertaken.
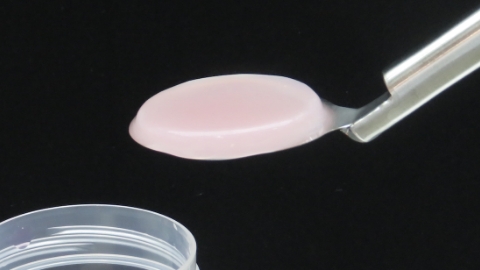
IK‐01 (Collagen gel matrix)

Dr. Ryosuke Kuroda
Professor, Department of Orthopaedic Surgery, Kobe University Graduate School of Medicine
Chairperson, The Japanese Knee Society (since 2023)
Director, The Japanese Orthopaedic Association (since 2021)
Auditor, Japan Sports Orthopaedic Association (since 2023)
Medical Committee Member, Japan Tennis Association (since 2023)
Team doctor, Orix Buffaloes, Vissel Kobe, Kobelco Kobe Steelers, and other teams
Promoted an investigator-initiated trial (from 2012 to 2016) in collaboration with FBRI to explore the safety and efficacy of the regenerative medicine for knee cartilage using IK-01 transplantation. Also participated as a medical expert in subsequent confirmatory trial and clinical trials in knee osteoarthritis.

Dr. Yasuyuki Ishibashi
Professor, Department of Orthopaedic Surgery, Hirosaki University Graduate School of Medicine (since 2012)
Chairperson, Japan Sports Orthopaedic Association (since 2023)
Auditor, The Japanese Knee Society (since 2023)
Medical advisor, Kashiwa Reysol
Participated as a coordinating investigator and a principal investigator in a confirmatory trial in traumatic cartilage damage and an exploratory trial in knee osteoarthritis with IK-01.
Treatment strategies for
knee cartilage with IK-01
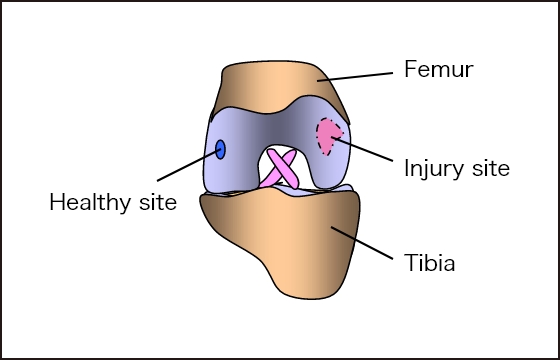
Autologous chondrocytes are collected
from healthy areas
Inside the 3D collagen matrix (scaffold)
Seed with autologous chondrocytes from
the healthy area
Cultured in CPC for 8-14 days
Short-term three-dimensional culture
(Maintains the cell's natural function)
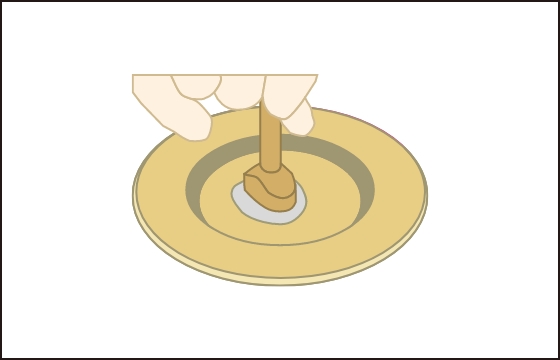
After the culture is complete, a special
instrument is used
to process the IK-01 according to the
injured area(2~9㎠)
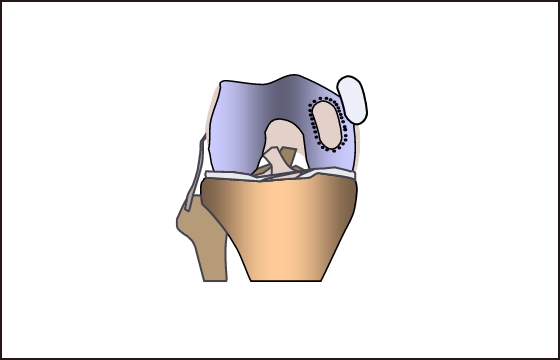
Using the same instrumentfor the
damaged area
Hollow out to the same size
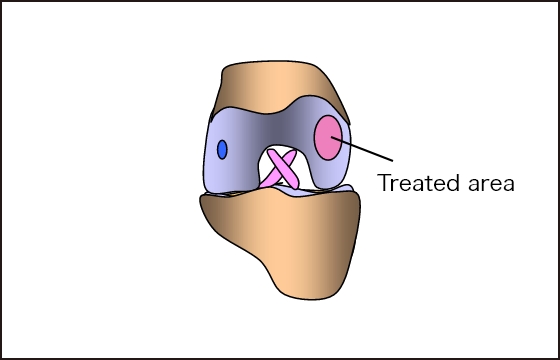
The processed IK-01 is transplanted into
the damaged area.
Implanted and fixed with fibrin glue
(no suturing required)
Excellent biocompatibility
(fits the injured site)
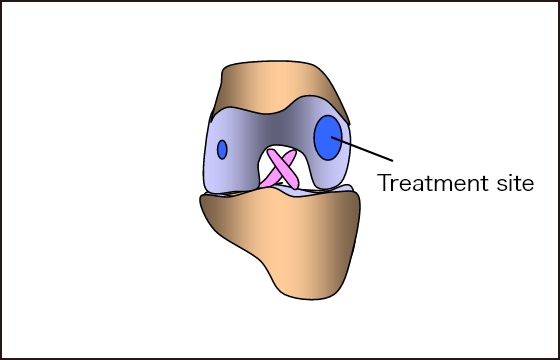
Contains type Ⅱ collagen
Regenerates normal hyaline cartilage
Expected long-term
effect

